25 Jun Niclosamide powder
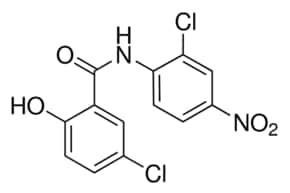
Niclosamide powder is a chemical compound with the formula C13H8Cl2N2O4. It is a member of the class of salicylanilides and has a cream color. It is used in the pharmaceutical industry as a USP and EMA standard. Analysis of the product’s purity is done using HPLC. The product is also available in a capsule form. Niclosamide is a non-hazardous substance and is incompatible with strong oxidizing agents.
Niclosamide powder is soluble in dimethyl sulfoxide and insoluble in water. It has a relative density of 1,615 g/cm3 at 20 ℃ at 1.013 hPa. This product is incompatible with strong oxidizing agents.
Niclosamide is a non-hazardous substance and is incompatible with strong oxidizing agents.
Niclosamide has been used as an anticancer drug in humans. Niclosamide has been shown to be effective at reducing fibrosis in patients with chronic liver diseases (cirrhosis and others). There was an advantage of niclosamide over the other drug that has been proven to be successful for cirrhosis fibrosis treatment; this is due to the fact that it does not cause myelosuppression. The non-myelosuppressive effect also makes it useful for treating solid tumors, especially when paired with a radiation therapy. Niclosamide has been shown to be effective at preventing nephritis in patients with interstitial cystitis.
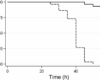
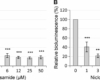
Niclosamide is used as a standard in the pharmaceutical industry for the purpose of analyzing the purity of the product. The purity and identity of the product is determined using High Performance Liquid Chromatography (HPLC). This purification technique is also used for analysis of other compounds such as sildenafil citrate, sildenafil, phenazopyridine hydrochloride, and others. Before using this compound to analyze purity, it should first be dissolved in 2M HCl for about 3 hours. The solution should then be allowed to sit undisturbed until crystals have formed. The resulting crystals should be dissolved in 10% HCl for about 2 hours. Solid impurities should be filtered out of the solution. The resulting solution should then be concentrated in the rotary evaporator until the volume is reduced to 1/3rd its original value. The final sample is ready for analysis using HPLC. In HPLC, the niclosamide powder tested should have a retention time of approximately 11 minutes, while impurity compounds have retention times longer than this time frame.
The discrimination of impurities can be done by using software or by having the sample tested in a HPLC device. There are several methods that can be used to accomplish this task. The most common method is simply by using a high performance liquid chromatography (HPLC) device. By using a chromatograph, it is possible to separate the molecules based on their retention times and identify impurities present in the sample. The use of this method is recommended for companies that intend to manufacture sildenafil citrate, sildenafil, and phenazopyridine hydrochloride.
Another method would be to use a paper chromatograph. This is a type of chromatography that uses a large number of paper filters in order to separate the mixture into different compounds. There are two types of filters used in this process. The first are is a supercritical fluid (SFC) and the second are solid phase extraction (SPE). The advantage for the SFC is that it allows the removal of unsaturated impurities with low water retention time and allows for the separation of dyes present in sildenafil citrate, sildenafil, and phenazopyridine hydrochloride. The SPE has lower pressure and allows for the separation of compounds with a high water retention time.
Using a paper chromatography is another method of purification and testing. This technique can also be used to analyze compounds in powder form like sildenafil, sildenafil citrate, and phenazopyridine hydrochloride. Di-isopropyl nitrite is not recommended for use in this method because it can damage the chemicals in the sample that are being analyzed.
https://pubchem.ncbi.nlm.nih.gov/compound/Niclosamide
Niclosamide powder is a clear product with the color ranging from white to cream colored. It has an acidic odor when viewed under an optical microscope. It is soluble in dimethyl sulfoxide and insoluble in water. The powder can be used to quantify the product. The sample of powder should be dissolved in 2M HCl for about 3 hours. After this time period, the liquid portion of the solution will separate and form a solid mass on the bottom of a cup, vial or beaker. This solid mass is called niclosamide and it can easily be collected from the solution using filter paper or a filter funnel.